
The Feeling of Having Another Pit in the Stomach – Part 2
The only way out of a hole is to clim out.
Cheryl Strayed
Hello back…
In this post, we conclude our discussion on duodenal perforations, focusing more on the surgical treatment.
Let’s not waste time and just get started…
Surgical Treatment
In patients with perforated peptic ulcer with significant pneumoperitoneum or extraluminal extravasation or signs of peritonitis, we recommend surgical treatment (Low-quality evidence-based recommendation, 1C).
We recommend surgery as early as possible, especially in patients with late presentation and patients older than 70 years (strong recommendation based on moderate quality evidence, 1B).
A 2013 cohort study from the Danish Clinical Registry of Emergency Surgery showed that, in the first 24 h after admission, each hour of surgical delay after hospital admission was associated with a 2.4% adjusted decrease in the probability of survival compared to the previous hour, over the entire observation period. Other studies highlight the importance of a rapid surgical approach to duodenal ulcer such as the single-centre retrospective study by Lunevicious et al. which showed that there is an increased rate of suture leakage after delayed presentation >9 h, while a recent prospective single-centre study of 101 patients with peptic ulcer perforation peritonitis undergoing laparotomy and simple omental patch closure found that a perforation-surgery interval greater than 36 h was significantly associated with increased postoperative mortality. In addition, a systematic review conducted in 2010 that included fifty studies with 37 prognostic factors comprising a total of 29.782 patients provided strong evidence of an association of advanced age, comorbidity, and NSAID or steroid use with mortality. Shock on admission, preoperative metabolic acidosis, tachycardia, acute renal failure, low serum albumin level, high ASA score, and preoperative delay >24 h were also associated with poor prognosis. Limiting preoperative delay, therefore, seems to be of great importance.
In stable patients with perforated peptic ulcer, we suggest a laparoscopic approach. An open approach is recommended in the absence of adequate laparoscopic skills and equipment (weak recommendation based on moderate quality evidence, 2B).
In unstable patients with perforated peptic ulcer, open surgery is recommended (strong recommendation based on very low quality evidence, 1D).
A recent meta-analysis by Cirocchi et al. compares laparoscopic surgery with open surgery in patients with perforated peptic ulcer: their study identified 8 RCTs for a total of 615 patients (307 patients undergoing laparoscopic repair and 308 patients undergoing open repair). The study concludes that the laparoscopic approach is associated with less postoperative pain in the first 24 h after surgery, fewer postoperative wound infections, and shorter hospital stay. No significant differences were found between laparoscopic and open surgery in terms of overall postoperative mortality, suture repair leakage, intra-abdominal abscesses, and re-operation rate. This is the strongest evidence in the literature and suggests that it is reasonable to opt for a laparoscopic approach in stable patients and in the presence of a skilled surgical team. The conversion rate to open surgery is low at approximately 7.9% and was due to large ulcers, difficult (posterior) location, association of haemorrhage, or patient intolerance to pneumoperitoneum. In addition, the Boey scale can help us to get an idea that patients with a score of 3 are candidates for open surgery (some authors even consider a Boey score of 3 to be a contraindication for surgery with a laparoscopic approach) by assessing the ASR > 90 mmHg, > 24 hours of perforation, and the ASA.
Therefore, according to the literature available to date, we can conclude that in stable patients and the presence of surgeons with experience in minimally invasive approaches, the laparoscopic approach is recommended with a level of evidence of 2B, while in unstable patients and/or in the absence of surgeon skills, open surgery is recommended.
In patients with perforated peptic ulcer less than 2 cm, we suggest primary repair. No recommendation can be given on the use of omental patch (weak recommendation based on low quality evidence, 2C).
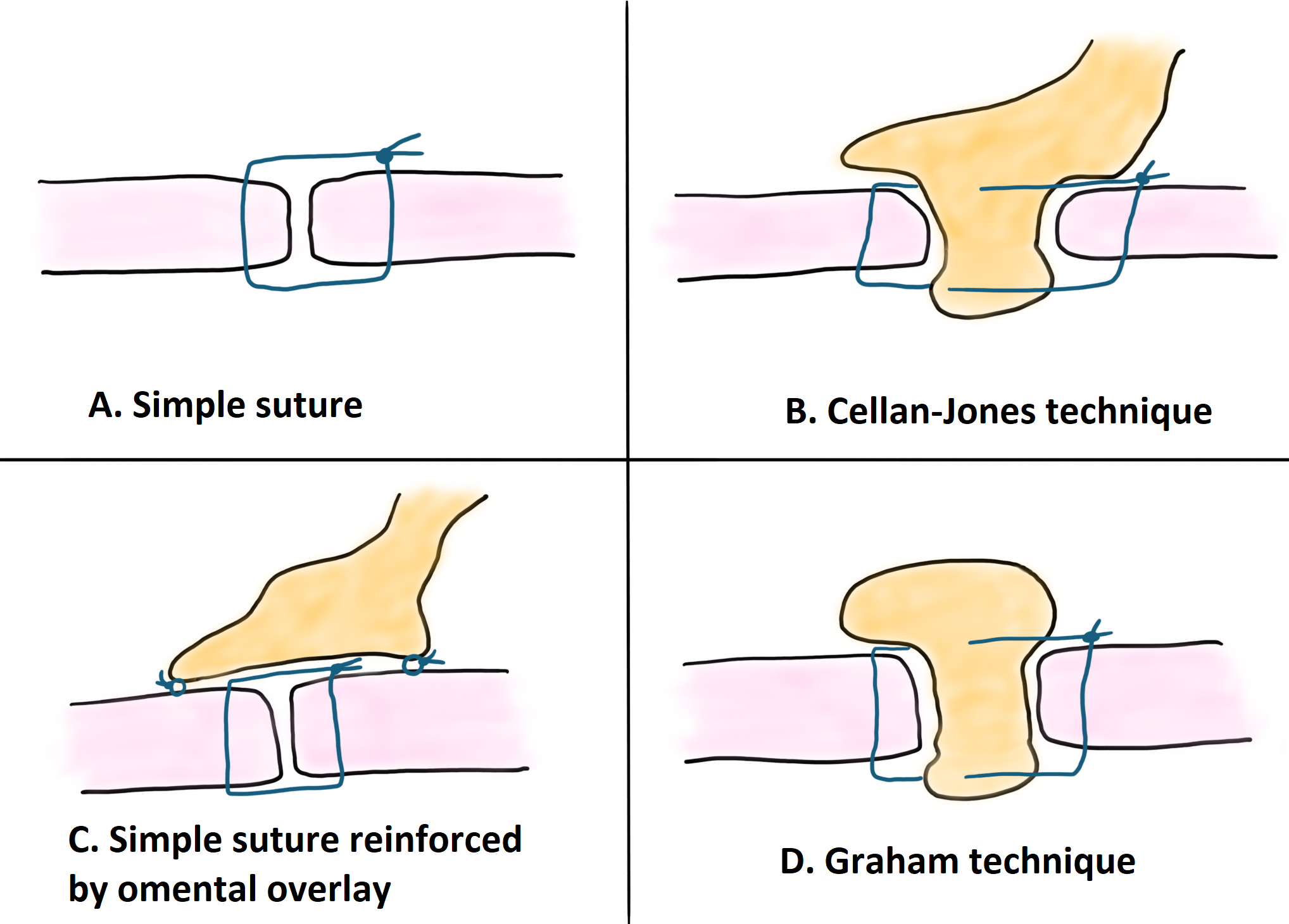
Historically, primary suture repair in conjunction with omental patching was considered the ‘standard’ laparoscopic procedure for perforated peptic ulcer repair. This belief is now the subject of debate, with multiple studies concluding that the addition of an omental patch does not add benefit to a simple suture, but significantly increases operative time.
Multiple studies support these findings. Lin et al. analysed 118 patients with PUs who underwent laparoscopic repair with simple closure (n = 27) vs omentopexy (n = 91). After matching, the simple closure and omentopexy groups had comparable results in terms of leak rate, but shorter operative time was evident in the simple closure group. Other studies come to the same conclusions.
On the other hand, other retrospective studies highlight the low postoperative leakage rates with the use of the omental patch technique, especially in cases of perforations larger than 2 cm in diameter. Therefore, there are articles suggesting the use of an omental patch for large ulcers with friable edges to reduce the risk of the suture cutting the edges of the ulcer. Because of the above, the routine application of the omental patch cannot be suggested due to the longer operative time, the need for advanced laparoscopic skills, and similar results after simple closure, but could be considered a viable option in selected cases (level of evidence 2C).
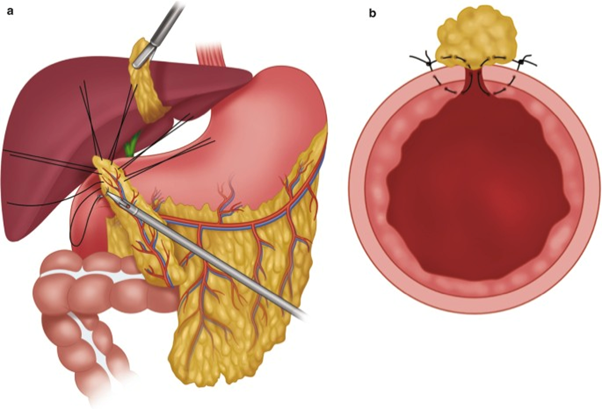
Primary suturing (transverse closure) can therefore be accompanied by the use of a pedicled omental patch (Cellan-Jones technique) or free omental patch (Graham technique).
We suggest a customised approach based on ulcer location for the treatment of perforated peptic ulcer larger than 2 cm. In case of large gastric ulcers raising suspicion of malignancy, we suggest resection with surgically frozen contextual pathological examination whenever possible. In case of large duodenal ulcers, we suggest considering the need for resection or repair plus/minus pyloric exclusion and external biliary drainage. We recommend duodenostomy only in extreme circumstances (weak recommendations based on very low quality evidence, 2D).
Large perforated duodenal ulcers pose a particular surgical challenge, as they are unlikely to be resolved by primary suture repair or omental patch repair, and outcomes are particularly poor if such a repair fails. Up to 12% anastomotic leakage has been reported if primary suturing is performed in duodenal ulcers >2 cm. In addition, they are associated with increased morbidity and mortality, higher leakage rates, and longer hospital stay. A large duodenal perforation is defined as any duodenal perforation that cannot be repaired by conventional methods due to the size of the perforation and the extent of native tissue loss (some authors draw the line at ulcers > 2 cm, others at 3 cm).
Before describing the different surgical techniques, we must take into account:
- The haemodynamic state of the patient: if the patient is unstable, damage control surgery should be performed;
- The location of the perforation;
- The diameter of the perforation (divide it into > or < 2 cm).
Different surgical techniques have been described, but there is still no clear consensus on which technique to use. Within these techniques, we can divide them into:
Techniques without resection:
- Free omental plug
- Triple tube
- Duodenojejunostomy
- Jejunal serosal patch
Techniques with resection:
- Antrectomy
- Partition of the gastric body
- Gastric resection
- Duodenectomy
The different techniques are presented below. The systematic review, including 25 studies on the treatment of duodenal perforation with large defects (i.e. >2 cm), has been used as a basis.
Omental plug
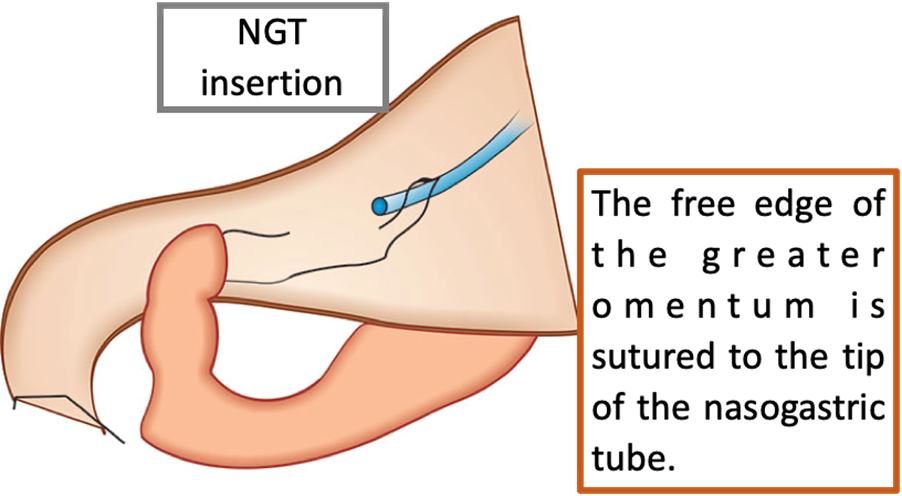
This technique was originally described by Karanjia in 1993. It consists of the insertion of a nasogastric tube, the tip of which is guided through the perforation. The free edge of the greater omentum is sutured to the tip of the nasogastric tube with an absorbable suture, then an omentum plug is pulled into the duodenum to occlude the perforation. The omentum and duodenum are then fixed. The nasogastric tube is removed one week later, after the sutures have dissolved. The authors propose that the main advantage of this technique over the classic omentum patch repair is that while a patch placed on the outside of the defect could be disrupted by increased intraluminal pressure, an omental plug has more continuous contact with the duodenal mucosa.
Regarding the results of this technique, Jani et al. published the results of a prospective randomised controlled trial of 100 patients with large (2-3 cm) perforated duodenal ulcers treated with classic omentopexy versus omental plug and concluded (with statistically significant results) that omental plug has a lower short and long term morbidity compared to standard omentopexy and a lower incidence of postoperative leakage. In addition, they also describe a lower incidence of posterior duodenal fistulae. No statistically significant differences in mortality were found.
The main advantages of the omental plug technique are that it is simple, quick, and accessible to any general surgeon or trainee surgeon in an emergency situation with a critically ill patient. Although the abnormal duodenal segment is left in situ, omental plugs have been shown to provide a near-normal duodenal mucosal surface. This type of repair does not involve diversion of gastric or duodenal contents outside the repair and does not require re-establishment of gastrointestinal continuity at a later date.
The disadvantages are that it has only been described in observational studies and a single randomised clinical trial, and no statistically significant differences in mortality have been demonstrated.
Triple tube technique
Initially described in 1954 for closure of the duodenal end after gastrectomy. Subsequently, in 1979, Stone and Fabian introduced this technique for cases of traumatic duodenal perforation.
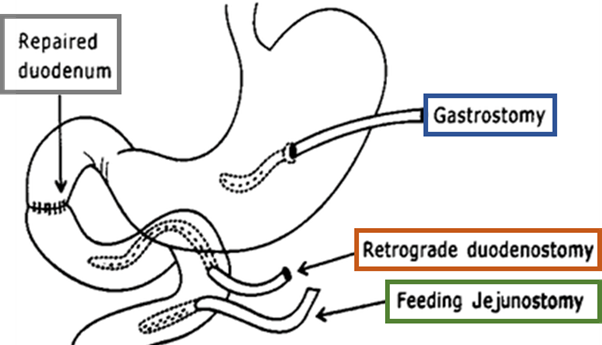
The triple tube technique involves performing the Kocher manoeuvre, excision of the ulcer margins, and primary closure of the duodenal perforation. Subsequently, three tubes are placed:
- First, an antimesenteric enterotomy is performed in the jejunum, approximately 15 cm distal to the duodenojejunal flexure, and a retrograde duodenostomy tube is placed, which allows decompression of the duodenum and removes tension from the primary repair.
- A second tube, an antegrade jejunostomy for feeding purposes, is passed through a second enterotomy 5 cm distal to the first.
- Finally, a gastrostomy tube is placed to reduce the secretion passing into the duodenum. This technique does not require the re-establishment of continuity.

There are only retrospective case-control studies describing a lower mortality in this technique compared to the simple omentopexy technique in duodenal ulcers >2 cm. This is due to a higher incidence of anastomotic leakage in patients undergoing omentopexy. In addition, they also describe a better hospital stay in this second group of patients. However, these results should be treated with caution as they are only retrospective studies.
Like the omental plug, the triple tube technique appears to be quick and simple, and can be performed by any general surgeon treating a patient in an emergency setting. However, it is dependent on the duodenal defect being amenable to primary closure. It also does not address concurrent problems, such as bleeding or gastric outlet obstruction, due to chronic scarring.
However, any comparison between these two groups should be made with caution, given the non-randomised design and the use of non-consecutive patients.
Furthermore, there are no clinical trials describing lower incidences of suture dehiscence with the use of this technique.
Gastric corpus partitioning
Two case series by Shyu et al. describe the gastric corpus partition technique for the treatment of giant perforated peptic ulcer. This technique involves simple closure of the perforation followed by partitioning of the gastric body with a linear stapler 2 cm proximal to the angular incisure. To re-establish gastrointestinal continuity, a gastrojejunostomy is performed, and a duodenostomy tube is inserted for biliary drainage. It is described that the partition should be performed in the gastric body and not in the antrum to reduce hypergastrinaemia.
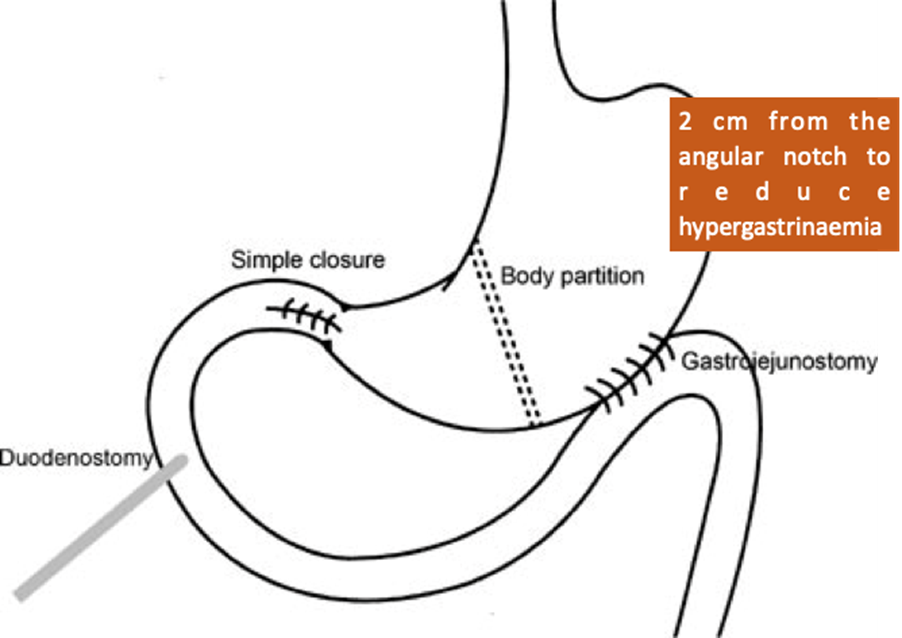
In two case series of elderly patients with good follow-up (2-3 years), they report a combined mortality of one in 18 patients (5.6%) and no major short-term complications. Although minor leaks occurred in seven out of 18 patients (38.9%), they were treated conservatively in all cases.
Gastric body partitioning is similar to the triple tube technique in that it relies on the primary defect being easily closed, but the bypass of the gastrointestinal contents is permanent and is achieved by simple stapling, which is much simpler than any form of resection but more complex than placing a gastrostomy tube. This technique requires the formation of a gastrojejunal anastomosis at the time of the initial surgery in order to resume oral intake.
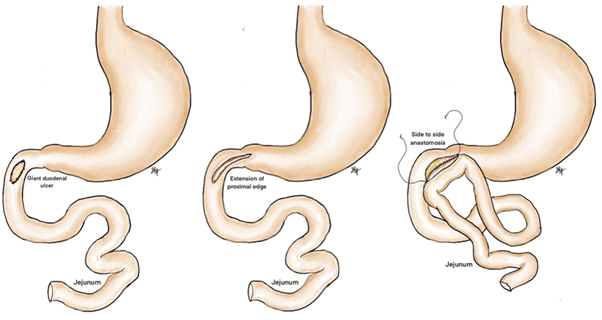
Duodenojejunostomy
In this technique, the perforation site is identified and extended towards the pylorus to minimise the risk of gastric outlet obstruction. Subsequently, a loop of jejunum is brought up retrocolically, and a manual side-to-side duodenojejunostomy is performed. In a small series of four patients, Gan et al. report no mortality and one case of postoperative pneumonia, with a mean length of stay of 11 days. The main attraction of this technique is its relative simplicity and, in theory, the use of a single anastomosis. Described in only one case series.
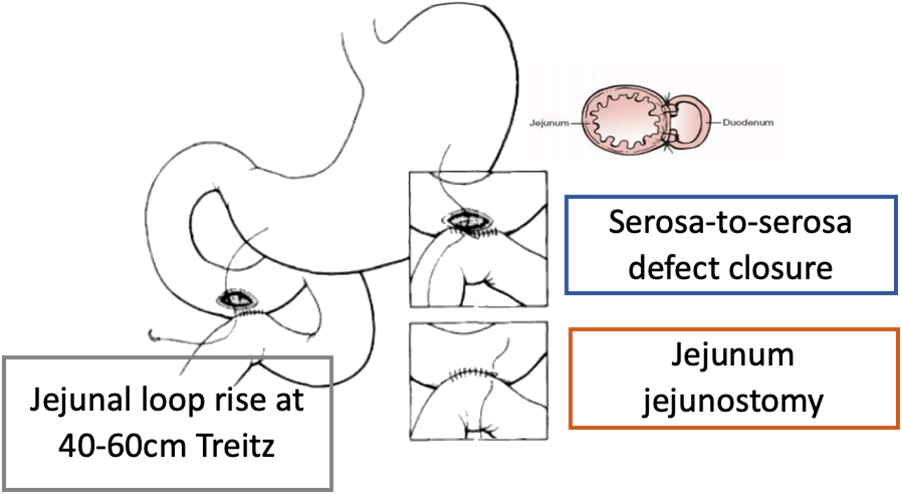
Serosa patch
This technique, originally described in animal models, involves bringing a loop of jejunum approximately 40-60 cm distal to the ligament of Treitz over the colon and using it to close the serosa perforation site to the serosa. A jejunojejunostomy bypass is performed. A case series by Chaudhary et al. on eight patients with large duodenal ulcer perforations, five of whom were treated using jejunal serosal patches, cites an overall 30-day mortality of three out of eight patients (37.5%) and an incidence of intra-abdominal abscess of four out of eight (50%) requiring reoperation in half of the cases. Unfortunately, the results of this case series were not broken down by type of procedure, making direct comparison with other techniques difficult.
In terms of our setting, this technique aims to close the duodenal defect by covering it with a serosal patch, and, over time, it has been shown that the duodenal mucosa extends over the serosal surface. The serosal patch may cause duodenal stricture or obstruction if used on large defects and is only recommended if one-half or two-thirds of the duodenal wall remains intact. The jejunojejunostomy is used for bypass, so it is not necessary to re-establish gastrointestinal continuity afterwards.
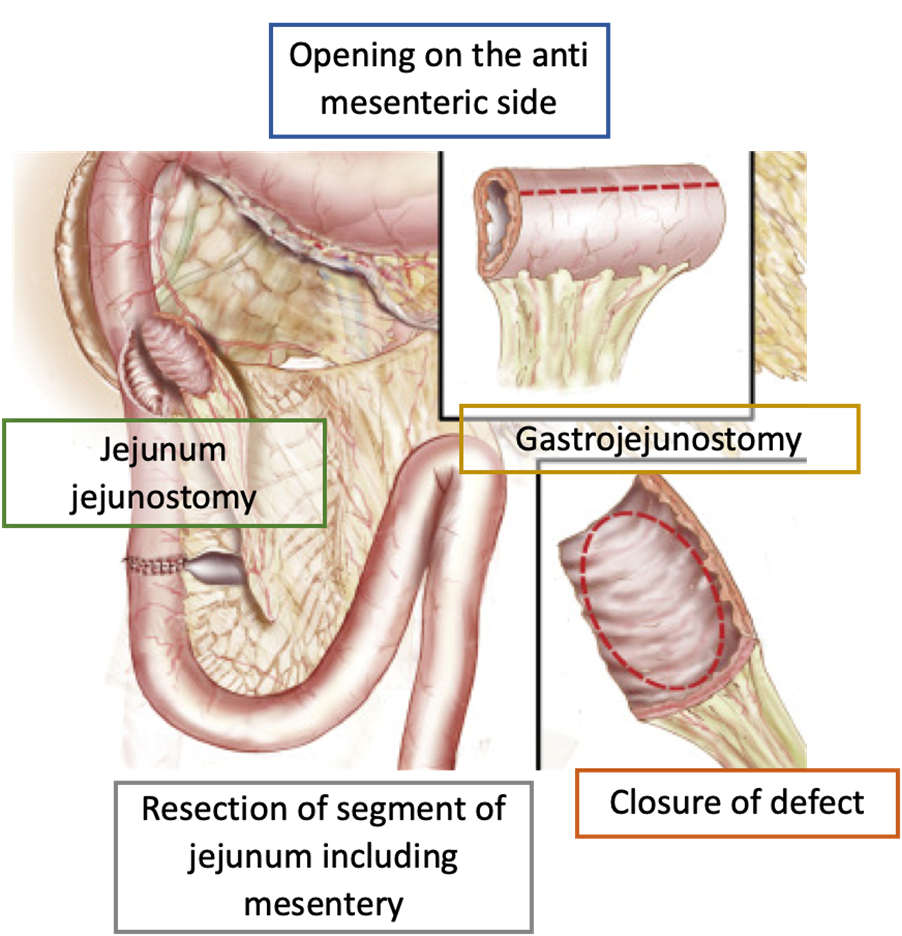
Pedicled jejunal grafting
This technique has been described to repair a large duodenal defect due to a perforated ulcer. A short segment of jejunum, including its mesentery, is resected approximately 20 cm distal to the ligament of Treitz.
This pedicled graft is brought through the transverse mesocolon, opened longitudinally along the antimesenteric border to cover the duodenal defect, before anastomosis. This technique requires a distal jejunojejunostomy at the graft harvest site and, in addition, the authors perform a gastrojejunostomy to partially bypass the duodenum.
The use of pedicled grafts may be beneficial in situations where little healthy duodenal tissue remains, as a pedicled graft may allow the transfer of more healthy tissue into the damaged duodenum, compared to the serosal patch, which may result in significant narrowing. However, this technique is complex, requires several anastomoses, and there are very few case reports describing its results.
Pancreas-sparing duodenal resection
Di Saverio et al. have recently presented a case series of 10 consecutive patients (seven of whom had giant perforated duodenal ulcers) treated with ampulla-sparing duodenectomy without pancreatic involvement for D1/D2 lesions proximal to the ampulla of Vater. This technique involves performance of the Kocher manoeuvre of the duodenum, cholecystectomy, and placement of a transcystic tube down the common bile duct into the duodenum. A distal gastrectomy is performed, which allows the specimen to be flipped and the proximal duodenum to be dissected from the pancreas. A tangentially hinged endograft is used through the healthy duodenum proximal to the ampulla. Gastrointestinal continuity is restored with a Roux-en-Y gastrojejunostomy, and external biliary drainage is achieved with the transcystic tube. This technique resembles a case described by Ntlhe et al.
Regarding intraoperative results, the authors report a mean operative time of 4 hours. The mean hospital stay was 17.8 days, and, with this technique, they reported a mortality of 20%, with a morbidity of 90%.
Although these techniques, in comparison to the previous techniques described, allow definitive management and can be used in extensive lesions that would otherwise require a pancreaticoduodenectomy, these procedures should be avoided at all costs in the emergency setting. It is a complex technique as the surgical time is approximately 4 hours, with an average hospital stay of 18 days and a mortality of 20% and a morbidity of 90%.
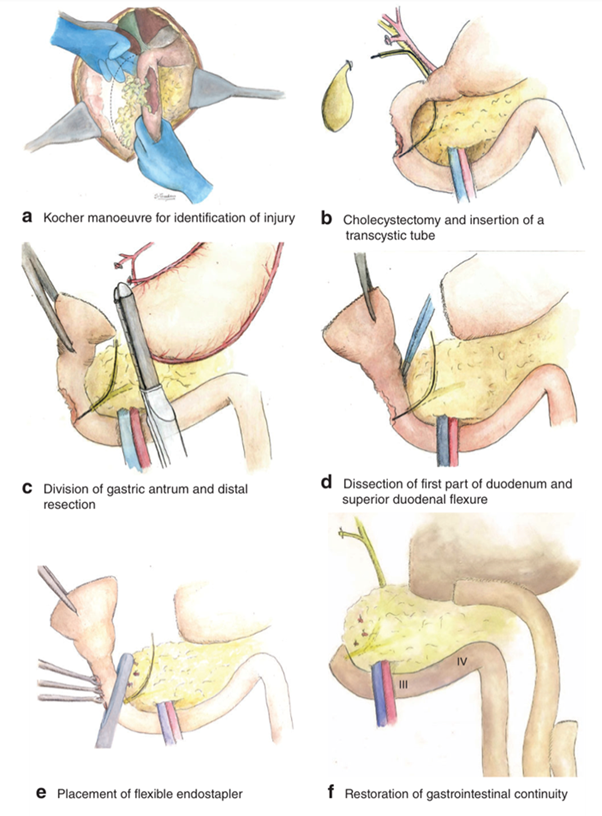
Gastroduodenal resection
Several articles present results after gastric resections for large perforated duodenal ulcers, the most common resection being Billroth II, but Billroth I and atypical resections are also cited in the literature. In a retrospective cohort study of 58 patients undergoing gastrectomy for large peptic perforations, Chan et al. observed a mortality rate of 20.7%. The mean operating time for this procedure was approximately 3 hours, with a mean hospital stay of 13.5 days.
Kim et al. describe successful laparoscopic treatment of giant perforated duodenal ulcers in a series of five patients, but it should be noted that the surgeon who operated in this case series had extensive experience in laparoscopic gastric resection.
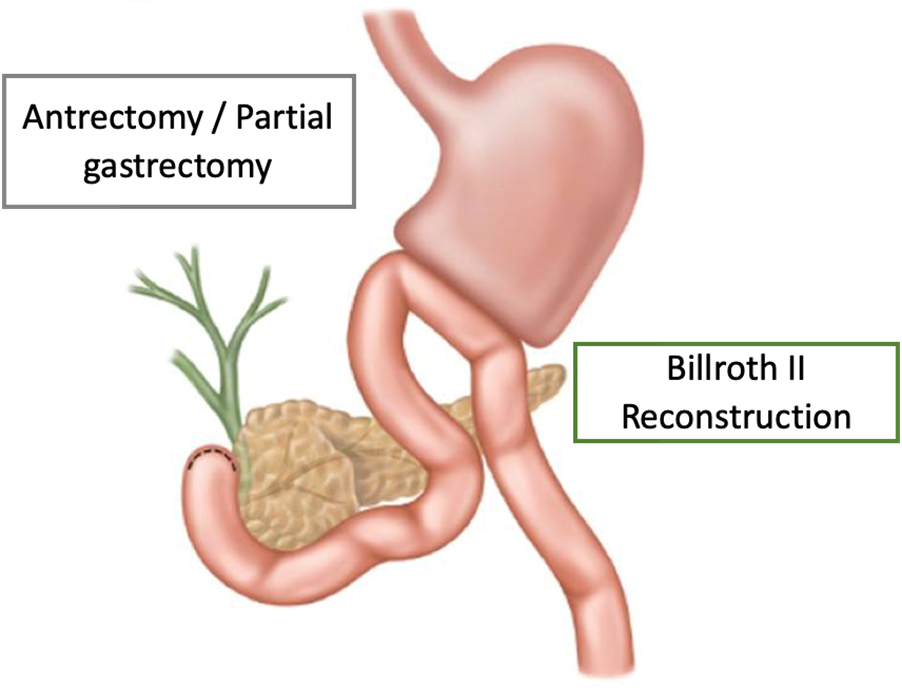
Pyloric exclusion
Traditionally used in the treatment of complicated duodenal injuries to temporarily protect the duodenal repair and prevent septic abdominal complications. The pyloric exclusion technique developed by Vaughan et al. in 1977 requires the pyloric muscle to be closed with staples or an absorbable suture. This is followed by a gastrojejunostomy for the gastric contents outlets. The stapled or sutured pylorus will open spontaneously in several weeks.
Seamon et al. reviewed retrospective studies reporting a trend towards a higher overall complication rate in the pyloric exclusion group (71% vs. 33%) and concluded that simple repair without pyloric exclusion was adequate and safe for most penetrating duodenal injuries.
Technique currently in disuse.
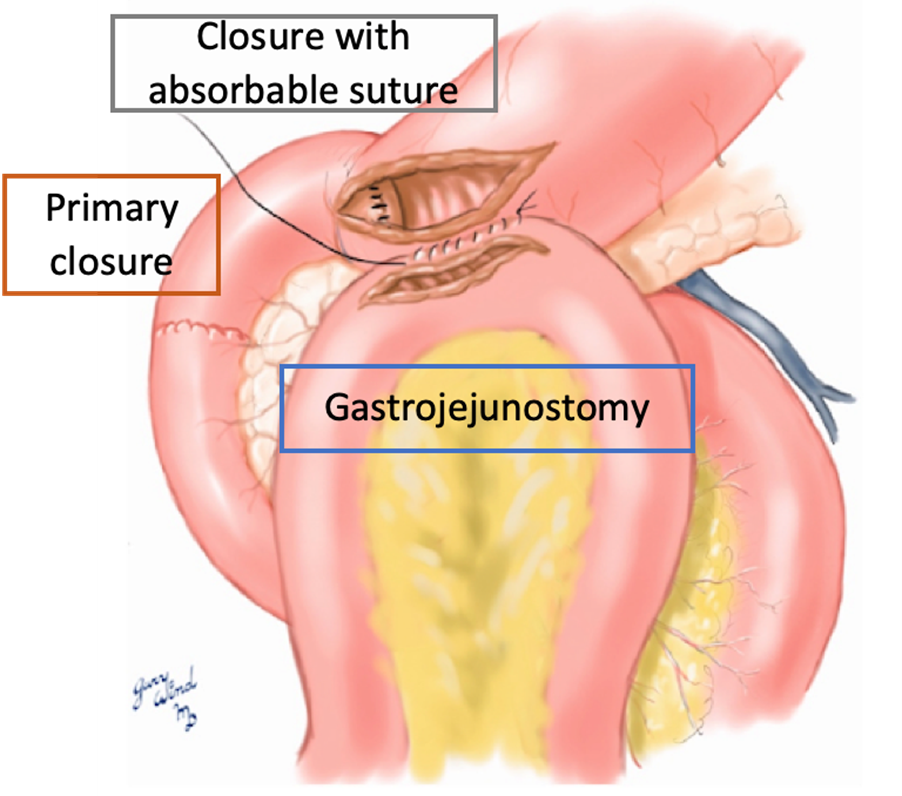
Giant Duodenal Ulcers
Currently, there is no consensus on the optimal surgical management of giant duodenal ulcer perforations. WSES guidelines suggest pancreas-sparing duodenectomy for ulcers in D1/D2. For perforations involving the ampulla, a definitive resection approach is not recommended. Instead, the guideline favours damage control options such as pyloric exclusion (although this technique is the most widely described in the trauma literature and only described in one case in the context of ulcer perforation), gastric decompression, and external biliary drainage.
Overall, the main limitation of the literature presented so far is that the evidence base for the surgical management of giant duodenal ulcer perforations is of low quality. With only one prospective randomised controlled trial, the vast majority of evidence comes from case reports or series as well as cohort studies, representing evidence levels 3b-4. In addition, there are no articles with a long follow-up. Due to the rarity of this pathology, institutions collect cases over many years to obtain a sufficient number of cases, which is why it is difficult to perform randomised clinical trials to compare different surgical techniques.
It is suggested that the problem be addressed in four distinct steps that require consideration:
- What is the best way to close, repair, or resect the perforated or damaged duodenum?
- Is diversion of gastric and duodenal contents necessary outside of this repair, and how best to achieve this?
- How and when to reconstruct gastrointestinal continuity if it has been disrupted?
- Consideration of access to enteral feeding distal to the perforation in these complex patients.
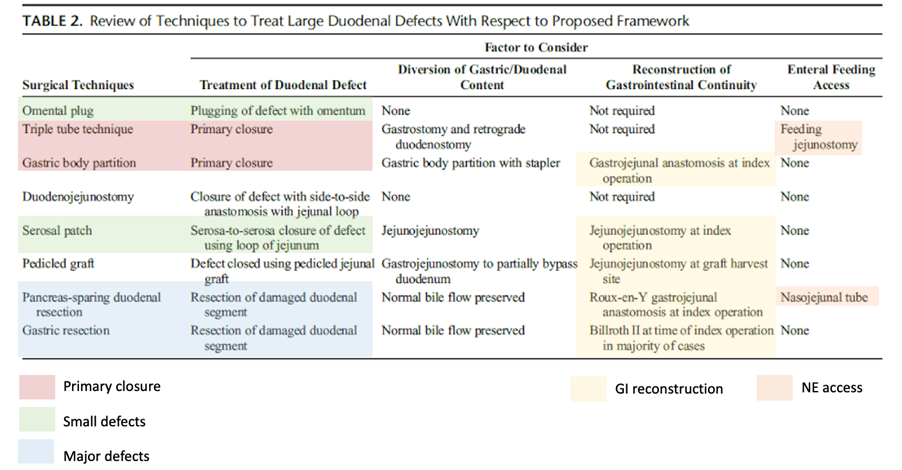
Table 2 (above) provides an overview of the extent to which the techniques reviewed address these four guiding principles and illustrates how the answer to each of the above questions will influence subsequent steps. Two of the techniques, the triple tube technique and gastric corpus partitioning, rely on the duodenal defect being amenable to primary closure. Similarly, serosal patching and omental tamponade require that part of the duodenal wall remains intact, whereas resection techniques such as gastrectomy and pancreas-sparing duodenal resection are not used when there is a greater degree of native tissue loss and inflammation.
Therefore, the degree of native tissue loss and inflammation will preclude the successful use of certain techniques. It should be noted that, of all the techniques reviewed, only the triple tube technique and pancreas-sparing duodenal resection explicitly describe a distal access for enteral feeding. Nevertheless, enteral feeding, ideally in the form of a nasojejunal tube or a feeding jejunostomy, is advised in all cases, as these patients are usually high-risk and often have not been optimised preoperatively due to the urgent nature of their intervention.
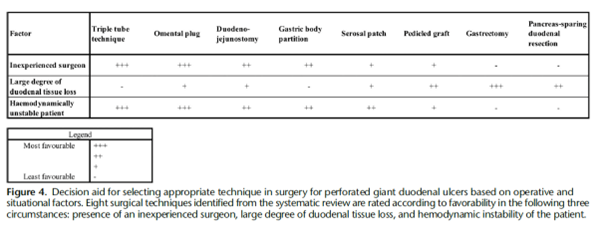
Figure 4 (above) presents a decision aid on the situational and operational factors that may inform the choice between these techniques. The technique chosen from this arsenal will depend on several factors, such as the location of the perforation (especially with respect to the bleb), the degree of duodenal leakage, the degree of contamination, the haemodynamic stability of the patient at the time of the intervention, as well as the surgeon’s experience. Although the current evidence does not support which surgical technique is superior in terms of morbidity and mortality, what is most important is a good understanding of the situation to allow the general surgeon to choose the best technique to perform on the patient.
Post-surgical Complications
It has been described that 30% of patients present with post-surgical complications of which include: surgical site infection, dehiscence, intra-abdominal collections, pneumonia, enterocutaneous fistula, peritonitis, incisional hernia, and paralytic ileus.
Mortality is a serious complication of PU. As mentioned above, PU carries a mortality ranging from 1.3% to 20%. Other studies have also reported a 30-day mortality rate as high as 20% and a 90-day mortality rate of up to 30%. Significant risk factors leading to death are the presence of shock on admission, comorbidities, resection surgery, female gender, elderly patients, a delay in presentation of more than 24 h, metabolic acidosis, acute renal failure, hypoalbuminemia, low BMI, and smoking. The mortality rate reaches 12%-47% in elderly patients undergoing PU surgery. Patients older than 65 years have a higher mortality rate than younger patients (37.7% vs. 1.4%). A study involving 96 patients with PUs also showed a nine-fold increase in postoperative complications in patients with comorbidities. In another large population-based study, patients with diabetes had a significant increase in 30-day mortality from PUs.
Treatment at Discharge
Persistence of H. pylori infection after simple closure surgery for duodenal ulcer perforation due to non-treatment of the duodenal ulcer has been shown to be an independent risk factor for ulcer recurrence.
The meta-analysis by Tomtitchong et al. described that the pooled 1-year incidence of ulcer recurrence in the H. pylori eradication treatment group was 5.2% (95% CI 0.7, 9.7), significantly lower than 35.2% in the control group (95% CI 0.25, 0.45).
Eradication of Helicobacter pylori after simple closure of duodenal ulcer perforation gives better results than simple closure plus anti-secretory treatment without eradication for the prevention of ulcer recurrence.
Therefore, at discharge, it is recommended to complete eradication treatment for H.Pylori.
In addition, a gastroscopy should be performed 4 weeks after completing the eradication treatment to assess the ulcer and take biopsies to verify eradication (Grade of recommendation A).
Discrepancies
Use of antifungals: we suggest not administering antifungals as standard empirical treatment in patients with perforated peptic ulcer.
In the case of perforated peptic ulcers, the high prevalence of fungal-positive cultures in peritoneal cultures and the associated poorer outcomes previously reported when present (according to previous literature), lead many clinicians to use empirical antifungal therapy in these patients in an attempt to decrease the risk of surgical site infections and even mortality. Interestingly, this practice does not seem to reduce the risk of perioperative complications and death. In a retrospective multicentre case-control study (554 patients), they studied whether the use of antifungal therapy is associated with a lower rate of surgical site infections (described as intra-abdominal collections, purulent discharge from the drain, or positive surgical culture in the first 30 postoperative days) and mortality. Fungal isolates are frequently found in peritoneal cultures obtained at surgery. However, the use of antifungals empirically is not associated with a reduced risk of postoperative surgical site infection, even when Candida spp was present. Both the World Society for Emergency Surgery and the Surgical Infection Society recommend the use of antifungal therapy empirically in high-risk patients (characteristics of these patients are detailed below); however, these recommendations are based on weak evidence and are not specific to patients with peptic ulcer perforations.
Antifungals should be administered to patients at high risk of fungal infection: immunosuppressed, advanced age, comorbidities, prolonged ICU stay, and unresolved intra-abdominal infections.
In conclusion, the use of empirical antifungals in patients with duodenal perforations varies significantly between surgeons and institutions, highlighting the lack of knowledge regarding the appropriate use of these agents. In this study, the use of this empirical treatment does not appear to provide any significant clinical advantage in the prevention of surgical site infections. The routine use of empirical antifungals in this setting should be discouraged. Further studies are needed to identify subgroups of patients who may benefit from the use of antifungals.
We hope this excursus on the perforated duodenum has been helpful.
See you next time…
References
- Barmparas G, et al. Empiric antifungals do not decrease the risk for organ space infection in patients with perforated peptic ulcer. Trauma Surg Acute Care Open 2021;6:e000662.
- Tarasconi A, et al. Perforated and bleeding peptic ulcer: WSES guidelines. World Journal of Emergency Surgery 2020;7:15:3.
- Songne B, et al. Non operative treatment for perforated peptic ulcer: results of a prospective study. Ann Chir 2004; 129:578-82.
- Chung KT, et al. Perforated peptic ulcer – an update. World J Gastrointest Surg 2017;9:1-12.
- Buck DL, et al. Surgical delay is a critical determinant of survival in perforated peptic ulcer. Br J Surg 2013;100:1045-9.
- Joshi MA, et al. Treatment of duodenal peptic ulcer perforation by endoscopic clips: A novel approach. J Dig Endosc 2017;8:24.
- Bergstrom M, et al. Self-expandable metal stents as a new treatment option for perforated duodenal ulcer. Endoscopy 2013;45:222-5.
- Bucher P, et al. Results of conservative treatment for perforated gastro-duodenal ulcers in patients not eligible for surgical repair. Swiss Med Wkly 2007;137:337-44.
- Lunevicius R, et al. Risk factors influencing the early outcome results after laparoscopic repair of perforated duodenal ulcer and their predictive value. Langenbecks Arch Surg 2005;390:413-20.
- Sivaram P, et al. Preoperative factors influencing mortality and morbidity in peptic ulcer perforation. Eur J Trauma Emerg Surg 2018; 44:251-7.
- Moller MH, et al. Preoperative prognostic factors for mortality in peptic ulcer perforation: a systematic review. Scand J Gastroenterol 2010;45:785-805.
- Cirocchi R, et al. Meta-analysis of perioperative outcomes of acute laparoscopic vs open repair of perforated gastroduodenal ulcers. J Trauma Acute Care Surg 2018;85:417-25.
- Lin BC, et al. Laparoscopic repair of perforated peptic ulcer: simple closure versus omentopexy. J Surg Res 2017;220:341-5.
- Abd Ellatif ME, et al. Laparoscopic repair of perforated peptic ulcer: patch versus simple closure. Int J Surg 2013;11:948-51.
- Lo HC, et al. Laparoscopic simple closure alone is adequate for low risk patients with perforated peptic ulcer. World J Surg 2011;35:1873-8.
- Gupta S, et al. The management of large perforations of duodenal ulcers. BMC Surg 2005;5:15.
- Siow SL, et al. Laparoscopic repair of perforated peptic ulcers: the sutured omental patch and focused sequential lavage technique. Surg Laparosc Endosc Percutan Tech 2014;24:134-9.
- Clinch D, et al. Duodenal ulcer perforation: A systematic literature review and narrative description of surgical techniques used to treat large duodenal defects. J Trauma Acute Care Surg 2021;91:748-58.
- Jani K, et al. Omental plugging for large-sized duodenal peptic perforations: a prospective randomized study of 100 patients. South Med J 2006;99:467-71.
- Bardou M, et al. Prise en charge thérapeutique de l’éradication de Helicobacter pylori chez l’adulte et chez l’enfant. APSSAPS; 2005.
- Tomtitchong P, et al. Systematic review and meta-analysis: Helicobacter pylori eradication therapy after simple closure of perforated duodenal ulcer. Helicobacter 2012;17:148-52.
- Bolaji T, et al. Management of the complex duodenal injury. Am J Surg 2023;225:639-44.
- Chung KT, et al. Perforated peptic ulcer – an update. World J Gastrointest Surg 2017;9:1-12.
- Cirocchi R, et al. A systematic review of the management and outcome of ERCP-related duodenal perforations using a standardized classification system. Surgeon 2017;15:379-87.
How to Cite This Post
Galofré C, Protti GP, Bellio G, Cioffi SPB, Marrano E. The Feeling of Having Another Pit in the Stomach – Part 2. Surgical Pizza. Published on July 26, 2025. Accessed on December 29, 2025. Available at [https://surgicalpizza.org/emergency-surgery/the-feeling-of-having-another-pit-in-the-stomach-part-2/].




